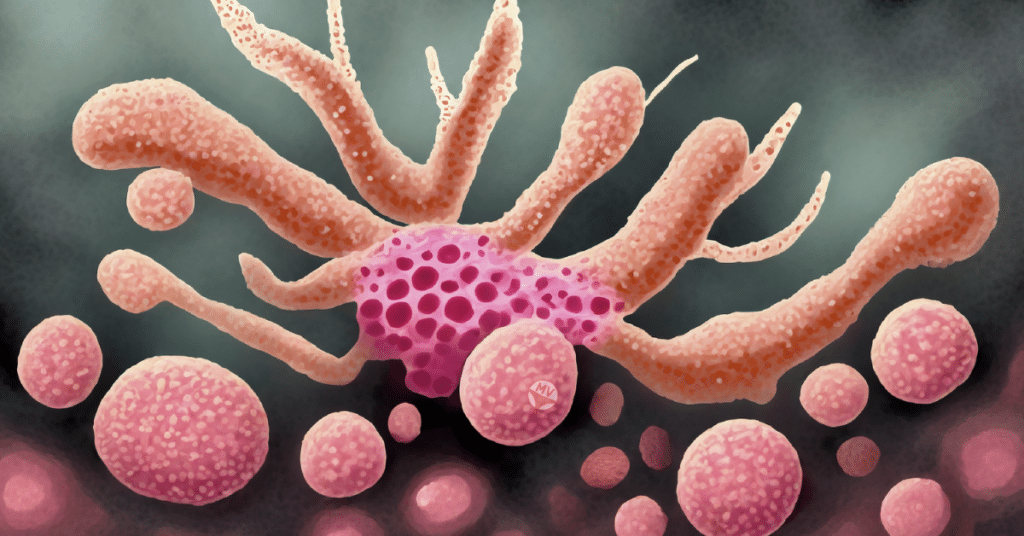
Oral and vaginal candidiasis, the bane of many mouths and vaginas, is a common opportunistic infection that brings discomfort, distorted taste sensations, mouth pain, and vaginal irritation, inflammation and pain.
When this infection takes hold in individuals with weakened immune systems, it can escalate into more severe complications such as systemic candidiasis and oesophageal candidiasis.
Candida albicans, followed closely by Candida glabrata, are the leading culprits behind oral and vaginal candidiasis. The problem arises when C. albicans erects its biofilm fortress in the oral or vaginal cavity.
This biofilm stands as a formidable adversary, resistant to conventional treatments. It deftly thwarts the penetration of antifungal agents, thanks to its extracellular DNA and EPS (extracellular polymeric substances).
To make matters worse, we’re now facing azole-resistant strains of Candida, and certain species are showing decreased susceptibility to antifungals due to overuse.
In light of this challenge, recent research has turned to Cinnamomum from cinnamon to wage war against Candida biofilms, thus reducing the selective pressure that breeds antifungal resistance.
A recent study1 unveiled Cinnamomum oil’s impressive potential in obliterating mature C. albicans biofilms that thrive on dental devices made from heat-polymerized polymethyl methacrylate (PMMA) resin. PMMA has a reputation for causing severe candidiasis and oropharyngeal issues in its wearers.
Cinnamomum oil impressively annihilated a staggering 99% of pre-existing Candida biofilm. Moreover, when PMMA samples were coated with this oil for a mere 24 hours, C. albicans biofilm formation was significantly curtailed by almost 70.0%.
Another investigation highlighted the prowess of C. burmannii essential oil and its aqueous extract, brimming with proanthocyanidins (Cinnulin), in deterring fungal adhesion to oral epithelial cells and putting up a strong resistance against preformed C. albicans biofilms.
Cinnamomum fractions contributed to bolstering the integrity of the oral epithelial barrier and displayed no toxicity to oral epithelial cells at their effective concentrations. Furthermore, Cinnulin dampened the secretion of interleukin (IL)-6 and IL-8 by oral epithelial cells provoked by TNF-α (Veilleux and Grenier, 2019).
So, it appears that different fractions of Cinnamomum could serve as practical allies in thwarting C. albicans biofilms, thereby helping to manage infections like Candida-infected oral mucositis lesions, oral candidiasis, and denture stomatitis, along with vaginal candidiasis.
Moreover, using these substances to coat dental devices could serve as a preventive measure against Candida biofilm formation, but more focused studies are warranted.
Freshly published research has also shed light on cinnamaldehyde’s potent antifungal properties against Candida species isolated from oral candidiasis patients.
Notably, cinnamaldehyde significantly reduced the biomass and metabolic activity of mature biofilms2. This reduction in biofilm biomass could play a pivotal role in taming multidrug-resistant infections since biofilms act as breeding grounds for cells endowed with advantageous traits such as creating new biofilms and enhancing virulence and adhesion.
Besides cinnamaldehyde, eugenol, another vital component of Cinnamomum, has also emerged as a formidable opponent against Candida biofilms.
Eugenol, a phenylpropanoid found abundantly in aromatic plants, particularly in clove oil, has demonstrated remarkable antifungal might against pre-formed Candida biofilms in a 2020 study3.
Exposure to eugenol led to the deformation of cell walls and the leakage of intracellular materials within Candida biofilms. What’s even more reassuring is that eugenol, at the concentrations tested, exhibited no cytotoxicity towards human non-cancerous keratinocytes.
Gas chromatography-mass spectrometry (GC-MS) analysis identified eugenol as the principal component of Cinnamomum essential oil.
Wijesinghe et al.4 further confirmed that eugenol constituted the majority (77.22%) of Cinnamomum essential oil. This essential oil significantly impeded the germ tube formation, adhesion, and biofilm construction of various common Candida species strains.
Microscopic examinations divulged that treatment with Cinnamomum essential oil resulted in the leakage of intracellular materials, cell wall damage, deformities, and a decline in cell density within biofilms.
Remarkably, Galleria mellonella larvae, used as an experimental model, showed no signs of cytotoxicity when exposed to Cinnamomum essential oil.
In another study5, CZEO (Cinnamomum zeylanicum essential oil) was found to suppress biofilm formation substantially and considerably diminish Candida monospecies as well as multi-species preformed biofilms at 24 hours and 48 hours, respectively. Eugenol was identified as the primary component (68.96%) of CZEO, confirming previous findings.
Additionally, this essential oil exhibited low cytotoxicity towards peripheral mononuclear and red blood cells.
On a different front, some studies have delved into the molecular interactions between Candida cells in biofilm communities and Cinnamomum, as well as cinnamaldehyde, yielding intriguing results.
For instance, a study6 by El-Baz et al. revealed that CVEO (Cinnamomum verum essential oil) possessed inhibitory effects against C. albicans biofilms isolated from various clinical samples. This essential oil also repressed the haemolysin and phospholipase activity of this fungus.
Microscopic images vividly displayed diminished biofilm formation, particularly in terms of adhesion suppression.
Intriguingly, molecular docking simulations indicated that cinnamaldehyde, as the primary constituent of CVEO, interacted with Als3, a significant adhesive protein in C. albicans. This finding is promising because the Als adhesive proteins are among the most thoroughly studied virulence factors in C. albicans, and disrupting them could significantly impair the fungus’s ability to adhere and form biofilms.
In a similar vein, another study7 uncovered that cinnamaldehyde disrupted Candida cellular growth and inhibited biofilm formation by affecting specific characteristics, including the expression of rare pseudo-hyphae and the absence of chlamydoconidia.
Molecular docking analysis revealed that cinnamaldehyde interacted negatively with certain cellular targets, with the highest affinity for squalene thymidylate synthase and epoxidase. This suggests that cinnamaldehyde could hinder biofilm formation in Candida by influencing crucial targets within fungal cells and nuclei, although further docking studies are warranted for precise identification.
Furthermore, Gupta et al.8 discovered that cinnamaldehyde could obliterate biofilm communities of C. glabrata clinical isolates from biomaterial surfaces such as contact lenses and urinary catheters.
Additionally, cinnamaldehyde augmented the production of ROS (reactive oxygen species), cell lysis, and the ergosterol content in plasma membranes. Interestingly, this compound suppressed the activity of C. glabrata enzymes such as phospholipase, catalase, and proteinase.
A detailed molecular analysis revealed that cinnamaldehyde downregulated the expression of various genes associated with 1,3-β-glucan synthase, sterol import, GPI-anchored protein, and multidrug transport.
The authors postulated that cinnamaldehyde’s interaction with ergosterol could alter the integrity and permeability of the cell membrane, leading to intracellular leakage and cell lysis.
In essence, the interaction between cinnamaldehyde and different Candida cellular pathways appears to suppress various virulence characteristics of this fungus, particularly within biofilm communities.
References
- 1.Choonharuangdej S, Srithavaj T, Thummawanit S. Fungicidal and inhibitory efficacy of cinnamon and lemongrass essential oils on Candida albicans biofilm established on acrylic resin: An in vitro study. The Journal of Prosthetic Dentistry. Published online April 2021:707.e1-707.e6. doi:10.1016/j.prosdent.2020.12.017
- 2.Miranda-Cadena K, Marcos-Arias C, Mateo E, Aguirre JM, Quindós G, Eraso E. Prevalence and antifungal susceptibility profiles of Candida glabrata, Candida parapsilosis and their close-related species in oral candidiasis. Archives of Oral Biology. Published online November 2018:100-107. doi:10.1016/j.archoralbio.2018.07.017
- 3.Marchese A, Barbieri R, Coppo E, et al. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Critical Reviews in Microbiology. Published online March 27, 2017:668-689. doi:10.1080/1040841x.2017.1295225
- 4.Wijesinghe G, Oliveira T, Maia F, et al. Efficacy of true cinnamon (Cinnamomum verum) leaf essential oil as a therapeutic alternative for Candida biofilm infections. Iranian Journal of Basic Medical Sciences. Published online May 2021. doi:10.22038/ijbms.2021.53981.12138
- 5.Rangel M de L, Aquino SG de, Lima JM de, Castellano LR, Castro RD de. In VitroEffect ofCinnamomum zeylanicumBlume Essential Oil onCandidaspp. Involved in Oral Infections. Evidence-Based Complementary and Alternative Medicine. Published online October 17, 2018:1-13. doi:10.1155/2018/4045013
- 6.El-Baz AM, Mosbah RA, Goda RM, et al. Back to Nature: Combating Candida albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics. Published online January 15, 2021:81. doi:10.3390/antibiotics10010081
- 7.da Nóbrega Alves D, Monteiro AFM, Andrade PN, et al. Docking Prediction, Antifungal Activity, Anti-Biofilm Effects on Candida spp., and Toxicity against Human Cells of Cinnamaldehyde. Molecules. Published online December 16, 2020:5969. doi:10.3390/molecules25245969
- 8.Gupta P, Gupta S, Sharma M, Kumar N, Pruthi V, Poluri KM. Effectiveness of Phytoactive Molecules on Transcriptional Expression, Biofilm Matrix, and Cell Wall Components of Candida glabrata and Its Clinical Isolates. ACS Omega. Published online September 28, 2018:12201-12214. doi:10.1021/acsomega.8b01856
Get a fresh perspective with a qualified, experienced vulvovaginal specialist naturopath.
This product has multiple variants. The options may be chosen on the product pageThe most comprehensive vaginal microbiome test you can take at home, brought to you by world-leading vaginal microbiome scientists at Juno Bio.
Easy-to-use BV and AV treatment program.
Promote and support a protective vaginal microbiome with tailored probiotic species.